Change the world
J2HBiotech - Journey to Healthcare Solutions
J2HBiotech - Journey to Healthcare Solutions
B-209~210,212 / B2-210~212
142-10, Saneop-ro 156beon-gil, Gwonseon-gu, Suwon-si, Republic of Korea



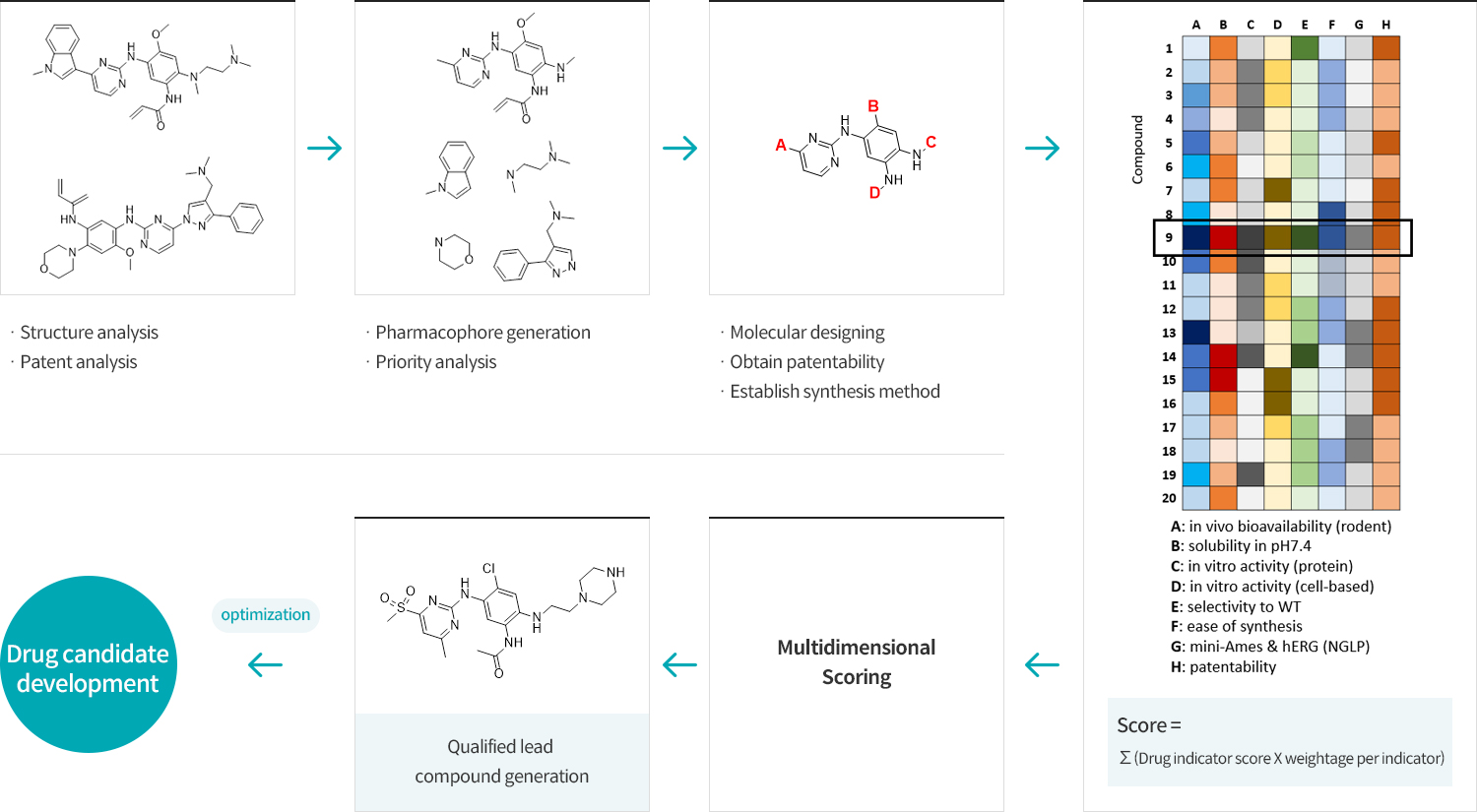
OPTIFLEX facilitates the new drug candidate discovery utilizing the multidimensional scoring system that evaluates and optimizes the physiochemical and pharmacokinetic properties to accelerate the derivation of the novel drug candidates




| Pipeline | Type | Target Disease | R&D Stage | ||||
|---|---|---|---|---|---|---|---|
| Discovery | Animal POC | Preclinical | Phase 1 | Phase 2 | |||
| J2H-1702 | Small molecule | Metabolic dysfunction- associated steatohepatitis | 50%
| ||||
|
|||||||
| J2H-1801 | Small molecule | Multiple sclerosis | 50%
| ||||
|
|||||||
| J2H-2002 | TPD | Non-small cell lung cancer | 50%
| ||||
|
|||||||
| J2H-2201 | TPD | Non-small cell lung cancer | 50% | ||||
| J2H-2104 | Small molecule | Solid tumor | 50% | ||||
| J2H-2103 | TPD | Solid tumor | 50% | ||||
| J2H-2202 | TPD | Non-small cell lung cancer | 50% | ||||
* TPD: Targeted protein degradation
Domestic Partners







Global Partners



Collaborative R&D







